Family: Surgeonfishes-Acanthuridae
Surgeonfishes get their name from the colored spine found on their caudal peduncle (the area right before the start of the caudal fin (tail fin)). It can be used as a slashing weapon to defend itself against predators. The scalpel-like blade is hinged in the back and held against the body inside a groove. When threatened it can be raised out of its groove and used as a formidable weapon. Most surgeonfishes are herbivorous and can often be seen traveling in schools scraping off algae found growing around the reef. They have developed thick stomachs to deal with the intake of calcareous materials that is ingested along with the algae.
This family of surgeonfishes consists of about 72 species in 6 genera, classified into two sub-families: the Nasinae, which are Indo-Pacific, and the Acanthurinae. The latter consists of three tribes: Prionurini, Zebrasomini, and Acanthurini. The tribe Acanthurini contains 42 species, 3 of which (in the genus Acanthurus) occur in Caribbean waters.
Surgeonfishes (also called ‘tangs’) have pelvic fins with 1 spine and 3 or 5 (usually 5) soft rays, a continuous dorsal fin with 4-9 spines and 19-31 soft rays, and an anal fin with 2 or 3 spines and 19-36 soft rays. They have a deep, compressed, oval-shaped body usually less than 25 inches in length, with symmetrically rounded anal and soft dorsal fins, a small terminal mouth, large eyes high on the head, spatulate incisor-like jaw-teeth, small scales, and emarginate caudal fins.
These tropical fishes are found in all warm marine waters, except the Mediterranean, and are quite numerous on shallow coral reefs.
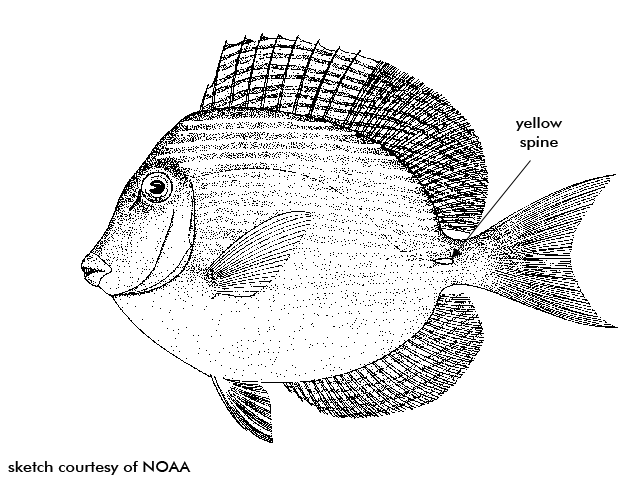
BLUE TANG Acanthurus coeruleus
pronounced a-can-ther-us so-roo-lee-us
Etymology: acantho, spiny or prickly; urus, from oura (= tail); coeruleus, blue
Description high and compressed bodies with a pancake like shape, with pointed snouts and small scales. Eyes are high on the head and mouth is small and low. Continuous dorsal fin. The distinct yellow caudal (tail) spines located at the base of the tail on both sides of the body is a distinct characteristic shared with other surgeonfishes. The spine actually fits within a horizontal groove and can be switched out and used as a weapon to fend of rivals and predators.
Blue tangs can be seen feeding by day in large schools mixed with other surgeonfishes. Blue tangs nip at the filamentous algae found on the reef, unlike the other surgeonfishes. They, therefore, have a thinner walled stomach because they do not have to digest the calcareous detritus. Blue tangs are generally blue in color with a yellow spine and are highly compressed.
They reach 40 cm in length and have 9 dorsal spines, 26-28 dorsal soft rays, 3 anal spines, and 24-26 anal soft rays.
These fish have three color phases: As juveniles, they are bright yellow. Adolescents change to a mixture of yellow and blue. As they mature, the color darkens to bright blue or purplish gray with a yellow caudal fin. The change to intermediate and adult coloration is not size-dependent, some yellow juveniles may be larger than the adult blue-phase. They reach sexual maturity in 9-12 months at 11-13cm in length. The sexes are separate (as opposed to hermaphroditic), they practice external fertilization, are nest guarders, and open water/bottom egg scatterers. The fish spawn in the later afternoon or evening. They typically change color from a uniform dark blue to a pale blue. Males aggressively court female members of the school, leading to a quick upward spawning rush towards the surface, where the sperm and eggs are released. The small, .8mm, eggs are pelagic and hatch in 24 hours, producing translucent larvae with silver bellies and tiny caudal spines.
Habitat Blue tangs are marine fishes found in the tropical Atlantic and Caribbean, where they are abundant. They hide in holes and crevices within coral reefs, where they find shelter from predators while they sleep at night.
Behavior Blue Tangs live singly, in pairs, or small groups, but sometimes they form large schools that forage on the reef, grazing on algae. Sometimes the schools include other surgeonfishes. Juveniles are rarely seen on reefs due to their small size and the need for cover. The intermediate phase is often seen on reefs. The yellow juvenile is very territorial, showing intolerance towards other yellow juveniles but not towards other species. It seems to ‘mellow’ with age, becoming a quite sociable adult that often mixes with other species when foraging. It tends to ignore divers, but withdraws slowly when approached.
Food Source/Strategy Blue Tangs feed entirely on algae. They are important in keeping algae growth on the reef under control, preventing the algae from overgrowing and suffocating corals. Juveniles also form cleaning stations, together with doctorfishes and sergeant majors, and graze algae and pick parasites and molted skin from the green sea turtle. The fish first does an inspection followed by feed nips on the turtle’s skin and shell. The most often cleaned body part is the turtle’s flippers. Unlike these relatives, the blue tang does very little bottom feeding or ‘scraping’ for algae from rocks and coral, which would bring considerable calcareous wastes along with the food. Instead, it nibbles on filamentous algae near reeftops, and thus has evolved a thinner-walled stomach.
Does another organism commonly hunt this fish? Blue tang predators include tunas, bar jacks, tiger groupers, and other larger carnivorous fishes. Larger fish are potential predators of the blue tang. Documented predators of this reef fish include mutton snapper (Lutjanis analis), tiger grouper (Mycteroperca tigris), yellowfin grouper (Mycteroperca venenosa), trumpetfish (Aulostomus maculatus), and the great barracuda (Sphyraena barracuda). The scalpel-like spine located on each side of the caudal peduncle is used as the primary defense mechanism against predators. These spines are used in a slashing motion to inflict serious wounds on the unfortunate victim. Predation pressures are highest on this species during the larval life stage in the open ocean.
OCEAN SURGEONFISH Acanthurus bahianus
pronounced a-can-ther-us bah-he-anus
acantho, spiny or prickly; urus, from oura (= tail); bahianus, from Bahia in the Florida Keys
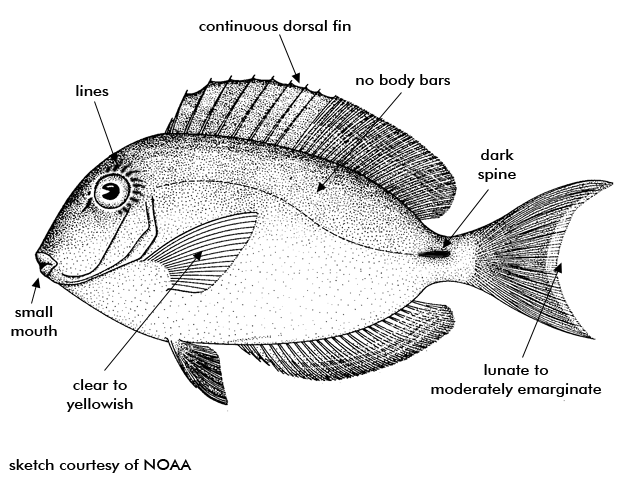
Ocean Surgeonfish feed during the day among other surgeonfishes on short algae that is scraped from the hard substrates by their teeth. They are generally a shade of light blue but can be dark. There is no barring on the body and the pectoral fins are transparent.
Description The oval-shaped body of the ocean surgeon is moderately deep and compressed. The mouth is small and low on the head, well adapted for scraping algae from rocks and coral. There is a sharp scalpel-like spine located on the side of the caudal peduncle that fits into a horizontal groove. The relatively long dorsal fin is continuous and unnotched. The caudal fin is moderate to deeply emarginated. The scales are small and ctenoid. The stomach is gizzard-like. There may be slightly darker narrow stripes on the body, alternating narrow bands of pale brown and turquoise on the dorsal fin, and bands of dark gray and gray-blue on the anal fin. The caudal fin can be lunate to moderately emarginate, usually similar to the body color but may have a pale area near its base. The dorsal, anal, and caudal fins have bluish-white margins; the pelvic fins have pale blue rays and black membranes; the pectoral fins are clear to yellowish. The peduncular spine has a black-edged dark brown sheath surrounded by violet. The rear edge of the opercle is blackish, while the rear edge of the eye has 6 or 7 thin yellow lines radiating outwards. It is similar in appearance to A. chirurgus (doctorfish), which differs primarily by having body bars and dark brown pectoral fins with pale outer margins. It typically grows to 6-12 inches, sometimes reaching 15 inches.
Habitat Inhabiting shallow bottoms over coral and rock formations (15-80 feet), the ocean surgeon typically occurs in groups of five or more. It is primarily a diurnal species and often schools with other species including the blue tang and surgeonfish.
Behavior, Food Source/Strategy Ocean surgeonfishes are herbivorous, with incisor teeth that are well-shaped for scraping algae from rocks and corals and compacted sandy bottoms. They feed by day and their algae grazing is usually done by roaming the reefs in small groups or schools, often mixed with other species, but some are solitary and territorial, patrolling food regions that are 4 to 12 yards square. Their digestive tracts are long, as is typical for herbivores, and their stomach walls reflect their feeding environment: they feed on sandy bottoms and short algae usually ingest some sand, and therefore have evolved thick-walled gizzard-like stomachs.
It tends to ignore divers, but will withdraw slowly when approached.
Camouflage Abilities the Ocean Surgeonfish can actively change its coloration, varying from bluish-gray to yellowish/grayish/dark brown.
Does another organism commonly hunt this fish? Ocean Surgeonfish predators include tunas, bar jacks, tiger groupers, and other larger carnivorous fishes. Larger fish are potential predators of the ocean sugeon. Documented predators of this reef fish includemutton snapper (Lutjanis analis), tiger grouper (Mycteroperca tigris), yellowfin grouper (Mycteroperca venenosa), trumpetfish (Aulostomus maculatus), and the great barracuda (Sphyraena barracuda). The scalpel-like spine located on each side of the caudal peduncle is used as the primarily defense mechanism against predators. These spines are used in a slashing motion to inflict serious wounds on the unfortunate victim. Predation pressures are highest on this species during the larval life stage in the open ocean.
SYMMETRICAL BRAIN CORAL Diploria strigosa
pronounced die-plor-ee-ah stri-go-sa

This stony (hard) coral along with others gets its name looking like a brain. It is distinguished by other brain corals by its ridges equal in height and width, lack of a groove at the top of the ridge, and the long valleys are often connected. It also has a general symmetrical design look to it.
Description The symmetrical brain coral forms smooth flat plates or massive hemispherical domes up to 1.8 meters (5 ft 11 in) in diameter. The surface is covered with interlinking convoluted valleys in which the polyps sit in cup-shaped depressions known as corallites. Each of these has a number of radially arranged ridges known as septa which continue outside the corallite as costae and link with those of neighboring corallites. The ridges separating the valleys are smoothly rounded and do not usually have a groove running along their apex as does the rather similar grooved brain coral (Diploria labyrinthiformis). The coral has symbiotic dinoflagellate alga called zooxanthella in its tissues and it is these which give the coral its color of yellowish or greenish brown, or occasionally blue-grey. The valleys are often a paler or contrasting color.
Habitat Inhabit many marine environments, down to 40 m.
Abundant to common in Florida, Bahamas and Caribbean.
What eats the coral? Parrotfish, butterfly fish, angelfish, sea slugs, snails, worms and the crown-of-thorns starfish all eat coral.
Does anything live within the coral head? Many things may live within coral heads, possibilities include Christmas Tree Worms, Spirobrancheus giganteus as well as many other invertebrates.
Family: Lugworms-Arenicolidae
These burrow dwelling polychaete worms move in their burrow by use of peristaltic contractions. They dig a U-shaped burrow in the sediment with two openings. The openings are to allow a good flow of oxygenated water. One side of the tube is where the worm ingests the sand we the other opening is where the worm deposits its waste.

SOUTHERN LUGWORM Arenicola cristata
pronounced air-in-i-cola crease-ta-ta

This animal is very common on the fine-grained sandy bottoms. It will create mounds of sand. This sand is the waste product from its feeding. Periodically it will look like the mound is erupting like a volcano. This is caused by the worm forcing the waste out of its burrow.
Description The lugworm, Arenicola cristata, is a large cigar-shaped polychaete with 17 setae-bearing segments . The surface of the body is roughly textured and green to brownish-green in color. Three body regions are apparent when examining the appearance and behavior of the lugworm. Thefront region is a muscular portion primarily used for digging. Eleven pairs of bushy red gills cover the middle of the body, which controls respiration. Excretion occurs at the back, which is usually the only portion visible above the sediment when the worm is buried. The body is segmented, or ringed. The head end is dark red; behind it the body is fatter and lighter in colour. Toward the tail the body becomes thinner and yellowish red. The middle of the body has bristles and about 12 pairs of feathery gills.
Habitat The range of A. cristata extends on the Atlantic coast of the U.S. from Cape Cod to Florida, throughout the Caribbean and the Gulf of Mexico. Lugworms reside in U-shaped burrows they excavate in undisturbed sandy or muddy sediments on protected beaches and tidal flats. The anterior end of the burrow becomes depressed as the worm ingests surface sediment in search of organic material on which it feeds. A mound of waste material and undigested sand is deposited at the opposite end of the burrow . The worms can be found throughout the lagoon in the sediments of sheltered beaches and tidal flats.
Reproduction In the spring, the lugworm produces a gelatinous pink egg mass, nearly one meter long. The mass is connected at one end to the burrow opening, with the remainder swaying freely in the current like a streamer. Some smaller individuals in sandy sediments may produce a compact ovoid or spherical egg mass instead. Embryonic development observed in the laboratory for egg masses held at 21°C was documented at 4-5 days, with a hatching time of 1-2 days. The larvae of the A. cristata are lecithotrophic, subsisting off limited energy stores before developing to a point when they are obligated to feed. This developmental mode allows the larvae more dispersal time as plankton in the water column before they must find a suitable habitat and settle to the benthos, a period of about 2-4 days for most larvae.
Food Source/Strategy Lug worms are known as non-selective deposit feeders, consuming subsurface sediment of decayed organic matter (and ingest sang along with the food particles). They defecate undigested material at the entrance of their burrows in the form of fecal castings that resemble those produced by sea cucumbers. Like terrestrial earthworms, this feeding method serves the important function of tilling sediments to promote aeration of soils and decomposition of organic material.
Personal Observations Because it is an infaunal species, burrowing in and consuming the sediment, A. cristata is used as an indicator species for the presence and effects of environmental contaminants and pollutants reaching benthic marine habitats from sources such as coastal runoff and ship traffic.
Is it a common food source for another organism? Fishes and other invertebrates may attack the hind end of the lugworm as it exits the burrow. In such an event, A. cristata has the ability to cast off and regenerate these segments in order to avoid predation, much in the same way a lizard sacrifices its tail or a crab drops a leg to escape predators. Lugworm eggs masses are preyed upon by a variety of benthic organisms, such as the bruised nassa, Nassarius vibex
Is it symbiotic or parasitic with another organism? Lugworm egg masses are also known to have a symbiotic relationship with diatoms fouling the exterior of their cases. Studies have suggested that the masses supply substratum for settlement of the diatoms, while oxygen produced by the microscopic algae provide buoyancy for the eggs, lifting them off the sediment and protecting them from benthic predators such as the bruised nassa snail, Nassarius vibex.